Kohji MAEDA
Synchronization and propagation of potential oscillations in artificial membrane systems
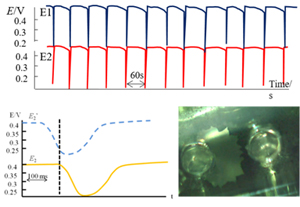
Cardiac, pancreatic and neuronal cells function as organs by synchronizing and propagating oscillations between multiple cells at the same time as the membrane potential oscillates. In our group, we are using artificial membrane systems such as liquid membranes to electrochemically analyze the potential oscillation response as a model for signal propagation and synchronization between cells.
Establishment of an artificial membrane model for energy conversion in cell membranes
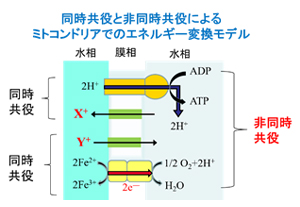
In energy conversions occurring in cellular membranes, such as respiration and photosynthesis, the conjugation of ion and electron permeation regulates the efficiency of energy conversion, but research focusing on this is limited. In our group, we are focusing on the conjugation of ion and potential membrane permeation, and using an artificial liquid membrane system, we are trying to obtain new knowledge about the principle of conjugation between the two and about the thermodynamics and kinetics of energy conversion.
Quantitative evaluation of diffusion of ions in various membranes

Membrane permeation of ionic molecules occurs when ions move vertically through membranes with various functional groups. However, when membranes are thin, it becomes difficult to analyze the vertical migration against the membrane. In our group, we have been analyzing the ion permeation through various membranes, such as cuticular membranes on the surface of fruits and Nafion membranes used as battery separators, using an electrochemical technique called as voltammetry for the ion transfer at the liquid-liquid interface.
Kinetic Analysis of Emulsions Using Electrochemiluminescence
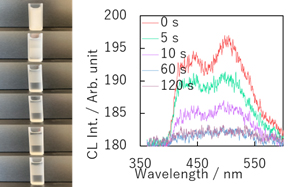
An emulsion is a metastable state created by dispersing immiscible solvents with a surfactant. As a result, the size and structure of emulsions change over time. Our group has succeeded in quantifying the ever-changing size of emulsions by using electrochemiluminescence. Using this method, we are attempting to analyze short-lived and unstable emulsions that have not been analyzed before.
Electrochemical measurement method of various ions using insoluble silver salts
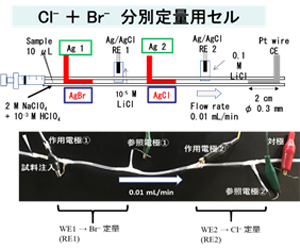
Anions have more limited analytical methods than metal cations. Silver ions form insoluble salts with various anions such as halide ions, sulfide ions, and arsenate and arsenite ions. We are developing an electrochemical quantification method for simultaneous determination of multiple anions, which has not been possible in the past, by utilizing the insoluble salt precipitation and elution reactions on the silver electrode surface.
Yumi YOSHIDA
Development of pont-of-care ion sensors
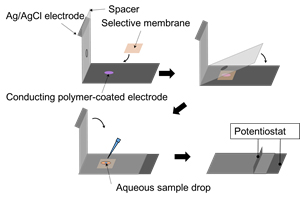
In telemedicine and automated production, rapid measurements are required at the patient’s side or in the field, not in the laboratory. Our group is developing a disposable ion sensor that can be used by non-analytical experts for accurate measurements. The use of ion-to-electron conduction converters (inorganic insertion materials and conductive polymers) has made it possible to produce ion sensors in large quantities using printing technology.
Coulometry for redox-inactive ions

Many analytical methods require a calibration curve using a standard sample. However, for valuable samples, it is necessary to perform the analysis under the condition that there are few or no standards for the calibration curve. In our group, we are developing an electrochemical method to measure the molar amount of ionic molecules in a sample of only 1 μL without using a calibration curve.
Elucidation of membrane permeation mechanism based on ion pair distribution model
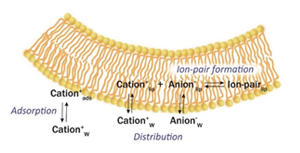
Since most drugs are ionic, there is a need for a membrane permeability theory that can predict the direct membrane permeation of ionic drugs in general physicochemically. In our group, we have developed a theory of membrane permeation of ionic molecules based on the assumption that cation-anion pairs are distributed in membranes (ion-pair distribution model), and have demonstrated this theory experimentally using lipid bilayers for the first time in the world.
Simultaneous measurement of the transmembrane current and the transmembrane fluorescent signal through a bilayer lipid membrane
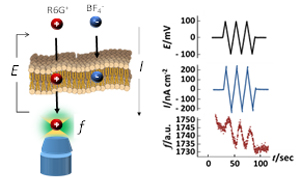
Ion permeation through lipid bilayers, the basic framework of cell membranes, has been analyzed by electrochemical measurements, in which a membrane potential is applied and the permeating ions are measured as an electric current. However, it has been difficult to determine which ions are permeating the membrane by electrochemical measurement. In our group, we have developed a fluorescence-electrochemical method that measures the transmembrane fluorescence signal as well as the transmembrane current, and have succeeded in analyzing the transmembrane permeation of fluorescently modified molecules.
Accumulation of ions to the inside aqueous phase of a liposome
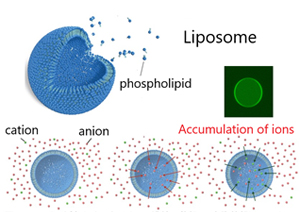
Based on the ion-pair distribution model, the membrane permeability of a target ionic drug can be controlled by changing the concentration of other coexisting ions. In our group, the concentration and release of ionic molecules into phospholipid vesicles (liposomes and vesicles) are realized by changing the ionic composition of the outer part of the vesicles. Currently, we are attempting to apply this technology to drug delivery methods for anticancer drugs. This technology has been applied for as an independent patent of Kyoto Institute of Technology.
